 |
CHEMISTRY Pre-AP Unit V: The Periodic Table Chapter 6 |
 |
Goal: The student will gain an understanding of the organization of the Periodic Table and the relationship between the periodic properties of the elements and their electronic structure.
Objectives: The student should be able to:
1. Discuss the contributions of Dobereiner, Newlands, Meyer, Mendeleev, and Moseley to the classification of the elements.
2. Discuss the significance of Mendeleev’s work and how it relates to the modern periodic table.
3. State the Periodic Law and explain the basis for the organization of the modern periodic table.
4. Define period, group, and family.
5. Explain the different systems for numbering the groups on the Periodic table.
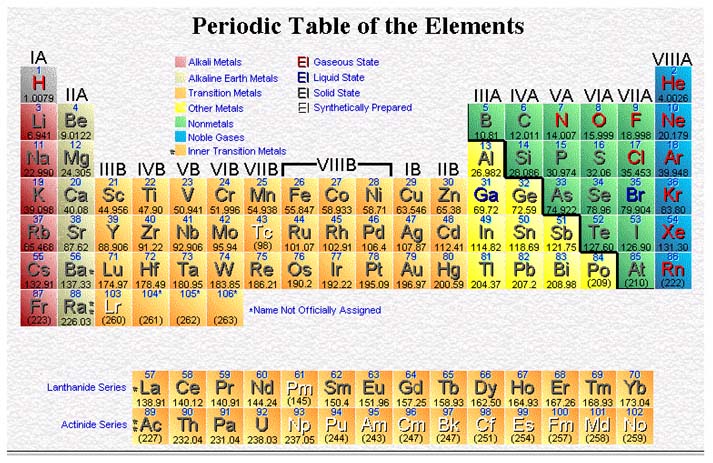
6. Name and locate on the periodic table: metals, nonmetals, metalloids, noble gases, alkali metals, alkaline earth metals, halogens, chalcogens, Lanthanides, Actinides, Transition metals, and Inner transition metals.
7. Apply the octet rule in predicting the stability of an element.
8. Predict the electron configuration of the outer energy level of any element from its position on the Periodic table.
9. Recognize exceptions to the Aufbau order due to half-full or full d-sublevels.
10. Define atomic radius, ionic radius, ionization energy, electron affinity, and electronegativity.
11. Predict trends in atomic radius, ionic radius, oxidation number, ionization energy, electron affinity, and electronegativity.
12. Explain trends in terms of nuclear charge and shielding effects.
Activities:
Oct 6: Self-Lecture Historical Development of the Periodic Table
Oct 11: Discuss Unit V Test; Lecture: Exceptions to Aufbau order, Ionization Energy, Electron Affinity
Oct 12: Lecture: Atomic and Ionic Radii, Electronegativity, Oxidation number
Oct 13: Lab: Group II Metals
Oct 14: Lecture: Chapter 7, The Elements
Oct 17: Discuss homework and Review for Exam V
Oct 18: Unit V Test
Lecture & Lab Materals:
Historical Development of the Periodic Table
The Modern Periodic Table and Exceptions to Aufbau